Products
R&D
Medical technology worldwide has been evolving at an accelerating pace.
Integrated efforts are leading to ever more sophisticated levels of medical innovation and technology.
Focusing primarily on innovation of therapeutic principles and enhancement of the treatment environment, we bring a novel approach to creating new and unique functions, researching the application of new materials, refining existing functions, and researching peripheral devices.
In order to cope with change and progress in medical technology, we research products that can form the core of future businesses and developing technology that can support these core businesses in the fields of both general surgery and dental treatment. At the same time, we carry on research and development in existing product improvement technology, production technology, and management technology.
We are currently working on research and development for new product development and existing product improvement in four different areas: surgical devices, ophthalmic devices, needles:eyeless・eyed, and dental devices. We are additionally engaged in patent management, IT system development, and sterilization and safety as common research themes.

In this area, we research and develop products used in surgery such as skin staplers and orthopedic surgical instruments. We carry out R&D from a long-term perspective, especially in the bone and cardiovascular fields, including research into new products for minimally invasive surgery and implants.
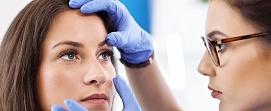
In this area, we research and develop products used in ophthalmic surgery such as ophthalmic sutures, ophthalmic knives, and other peripheral devices. We increase customer satisfaction by listening to user comments and responding to their requests through our research and development activities. We develop not only products used in vitreoretinal surgery but also a varied range of ophthalmic knives to meet the increasing market demand arising from the growth of microincision cataract surgery.

In this area, we research and develop mainly improved suture needles:eyeless・eyed. Our research targets include more sustained incision ability in running sutures, increased bending strength while maintaining safety, improved grip features on needle holders, and other user-friendly features.
We also research the relationship between the needle and the suture thread attached to it.
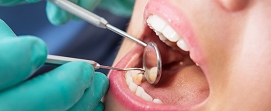
In this area, we research and develop instruments used in dental treatment such as root treatment tools, dental drills, dental sutures, and other peripheral instruments. We also develop dental prostheses and products related to endodontic and periodontic care.
We engage additionally in ongoing development of other existing therapeutic instruments and optic treatment instruments from a long-term perspective.