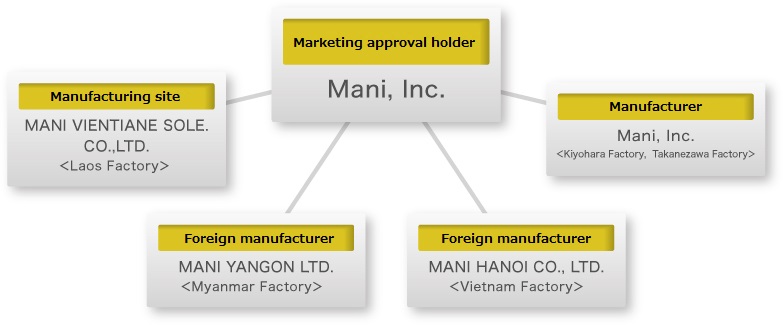
Products
R&D
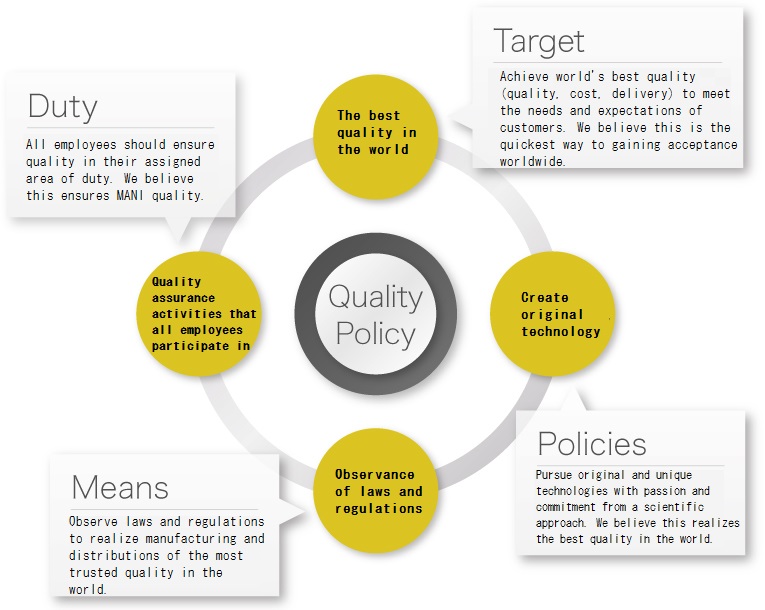
The best quality in the world (target)
Achieve world's best quality (quality, cost, delivery) to meet the needs and expectations of customers.Quality assurance activities that all employees participate in (duty)
All employees should ensure quality in their assigned area of duty.Creation of original technology (policies)
Pursue original and unique technologies with passion and commitment from a scientific approach.Observance of laws and regulations (means)
Observe laws and regulations to realize manufacturing and distribution of the most trusted quality in the world.
MANI has independently established high standards of quality and production. We plan to establish world-class management technology to maintain our production and information environment while further developing our original technology.
We acquire certification under external schemes such as ISO 13485 and maintain them through periodic audits.